BECAUSE ADRENOCHROME LEADS TO GEORGE SOROS: Fear, Anger, Hate, and Suffering Lead to Adrenochrome. ADRENOCHROME LEADS TO GEORGE SOROS. George Soros Leads to Fear.
BECAUSE ADRENOCHROME LEADS TO GEORGE SOROS: Fear, Anger, Hate, and Suffering Lead to Adrenochrome. ADRENOCHROME LEADS TO GEORGE SOROS. George Soros Leads to Fear.



ADRENOCHROME: The Blood Sacrifice Exposed
SOURCE CONSCIOUSAWARENESSFORALL NOVEMBER 10, 2014
Listen To This Article In AUDIO FORM:
What makes adrenochrome so valuable? It has psychoactive properties and can be used as a mind control drug. It can also be consumed to give someone an “adrenaline high.” Who would want adrenochrome? Former U.S. Vice President AL GORE was once apprehended at an airport with a suitcase full of packets of his own adrenochrome-laden blood. According to ALEX JONES, ALAN WATT and FRITZ SPRINGMEIER, all high-level bureaucrats and V.I.P.’s carry around at least 2 pints of their own adrenochrome-laden blood at ALL times.
Aside from mind control and euphoric properties, what else can adrenochrome do? Some believe that consuming the blood of a living creature steals its “life force” and transfers it to the drinker. Aside from “vampires,” many cults [Satanic and otherwise] are known for drinking human blood [“The Illuminati” would rank high in that list].
Why would Satanic and Illuminist cults want to drink human blood? ENTER: The Reptilian Blood Legacy. Whether you call them aliens, Annunaki, Nephilim, Chitauri, Dracos, Nagas or otherwise, many believe that our planet has long been infiltrated and ruled by extraterrestrial / extradimensional reptilian entities who manipulate global politics, business, banking, military and media.

Throughout history, we have seen numerous groups that have been dedicated to these reptilians: The Brotherhood Of The Snake, The Dragon Society, The Sons Of The Serpent, The Cult Of The Serpent, The Ophites, The Nergals, The Knights Of The Brazen Serpent, etc.Here are some images of ancient reptilian Annunaki statues:
These reptilians, possibly from the Draco or Sirius star constellations, are believed to be able to shape-shift. This could be done at will or because it is difficult for them to hold their form in this dimension / planet / atmosphere. I personally believe that it is hard for them to hold their form and that the consumption of blood, specifically adrenochrome, somehow gives them power and aids them in maintaining their desired form. I believe that this has been the main reason for blood sacrifices throughout history; not only giving the blood directly to the reptilians, but also performing the blood sacrifices in homage to them.
No Longer A Conspiracy Theory: Elite Openly Paying For The Blood Of The Young
By Matt Agorist
Once the talk of conspiracy theorists — the rich ingesting the blood of the young to foster longevity — is now a reality and an actual business in the United States. Not only is it a business but billionaires are actually admitting their interest in it.
“I’m looking into parabiosis stuff, which I think is really interesting. This is where they did the young blood into older mice and they found that had a massive rejuvenating effect,” Peter Thiel, the billionaire co-founder of PayPal and adviser to Donald Trump toldInc. magazine. “I think there are a lot of these things that have been strangely under-explored.”
But it’s no longer an experiment with just mice. The startup company by Jesse Karmazin, Ambrosia, is doing this with humans, and the rich are lining up to get the blood of the young.
As Vanity Fair reports, Ambrosia, which buys its blood from blood banks, now has about 100 paying customers. Some are Silicon Valley technologists, like Thiel, though Karmazin stressed that tech types aren’t Ambrosia’s only clients and that anyone over 35 is eligible for its transfusions.
As The Free Thought Project reported in January, a study published in Science and Nature Medicinerevealed that transfusing young mouse blood into old mice can actually prevent the symptoms of aging. This groundbreaking discovery could lead to medical breakthroughs and the development of new medicines. However, a report from the Vice health news outlet Tonic has pointed out far more sinister applications for this knowledge.
It was suggested in the report that aging elites are using the blood of young people as a type of youth serum. Now, we know that they actually are using it.
A similar claim was made by journalist Jeff Bercovici last year, after he conducted several interviews with Silicon Valley aristocrats including Peter Thiel, and learned about this transfusion procedure called “parabiosis,” where the blood of young people is used to prevent aging.
“There are widespread rumors in Silicon Valley, where life-extension science is a popular obsession, that various wealthy individuals from the tech world have already begun practicing parabiosis, spending tens of thousands of dollars for the procedures and young-person-blood, and repeating the exercise several times a year,” Bercovici reported.
In his article, Bercovici also expressed concerns about a developing black market for young people’s blood.
While there is certainly nothing wrong with willing young adults selling their blood to the elite, the underlying theme of this practice has strong roots in the occult.
In most modern cultures, mass murder and human sacrifice still takes place out in the open under the cover of warfare, while many argue that cannibalism also still takes place but behind closed doors.
It is only in the past few hundred years that the practice of cannibalism among royals has not been publicized. In Europe, around the time of the American Revolution “corpse medicine” was very popular among the ruling class, Charles II even brewed his own.

Dr Richard Sugg of Durham University has conducted extensive research into the practice of corpse medicine among the royalty.
“The human body has been widely used as a therapeutic agent with the most popular treatments involving flesh, bone or blood. Cannibalism was found not only in the New World, as often believed, but also in Europe,” Sugg said.
“One thing we are rarely taught at school yet is evidenced in literary and historic texts of the time is this: James I refused corpse medicine; Charles II made his own corpse medicine; and Charles I was made into corpse medicine. Along with Charles II, eminent users or prescribers included Francis I, Elizabeth I’s surgeon John Banister, Elizabeth Grey, Countess of Kent, Robert Boyle, Thomas Willis, William III, and Queen Mary,” he added.
If this wasn’t strange enough, the current royal family of England claims to be direct descendants of Prince Vlad III Dracula of Wallachia (modern Romania). This was the sick and depraved ruler, Vlad the Impaler, who was known as a butcher and who eventually became the inspiration for the most famous vampire stories in history.
Aside from the gruesome historical and occult background of such practices, there is a lack of data that suggests the process even works. Despite Karmazin’s claims that “young blood is causing changes that appear to make the aging process reverse,” scientists have yet to identify a link between blood transfusions from the young and any tangible health benefits.
“There’s just no clinical evidence [that the treatment will be beneficial], and you’re basically abusing people‘s trust and the public excitement around this,” Stanford University neuroscientist, Tony Wyss-Coray, who conducted a 2014 study of young blood plasma in mice, told Sciencemagazine last summer, as reported by Vanity Fair.
Adrenochrome Harvesting
SOURCE CONSPIRACYTHEORIESUSA NOVEMBER 30, 2017
This is what you find at the bottom of the rabbit hole after researching the latest sexual scandals in Hollywood and politics.
This video is not mean’t to be political in any way shape or form, all information reported has been sourced. Please watch the four videos below:
Whether human or animal, the blood sacrifice has long been a part of the rituals of many a civilization, past and present, here on Earth. From the Mayans to the Egyptians, the Jews to the Muslims, the Aztecs to the Chinese, the Zulu to the Greeks, virtually ALL civilizations have taken part in this ritual. But why? WHY do human beings feel that they can BENEFIT from the taking of another living creature‘s life?
Some blood sacrifices are supposedly performed to gain favor in the eyes of some deity, thus “ensuring“; a good harvest, good luck in the coming year or some other BULLSHIT like that. Some are done as punishment to those who have committed "evil" deeds, thus “restoring” the balance of good & evil in the universe. But I have discovered ANOTHER reason for the blood sacrifice: the extraction and collection of ADRENOCHROME.
What exactly is adrenochrome? In scientific terms, it is a chemical that is produced in the human body when adrenaline (epinephrine) oxidizes. How is the chemical extracted? A potential victim is terrorized, thus increasing the amount of adrenaline that is flowing through their body. They are then killed and the adrenochrome is collected with a needle and syringe from the base of the back of their neck and spinal column. Once collected, the chemical can be sold on the black market at exorbitant prices.
What makes adrenochrome so valuable? It has psychoactive properties and can be used as a mind control drug. It can also be consumed to give someone an “adrenaline high.” Who would want adrenochrome? Former U.S. Vice President AL GORE was once apprehended at an airport with a suitcase full of packets of his own adrenochrome-laden blood. According to ALEX JONES, ALAN WATT, FRITZ SPRINGMEIER, all high-level bureaucrats and V.I.P.’s carry around at least two pints of their own adrenochrome-laden blood at ALL times.
Aside from mind control and euphoric properties, what else can adrenochrome do? Some believe that consuming the blood of a living creature steal its “life force” and transfers it to the drinker. Aside from “vampires,” many cults (Satanic and otherwise) are known for drinking human blood. “The Illuminati” would rank high in that list.
Why would the Satanic and The Illuminist cults want to drink human blood? Whatever you call them aliens, Annunaki, Nephilim, Chitauri, Dracos or otherwise, many believes that our planet has long been infiltrated and ruled by extraterrestrial who manipulate global politics, business, banking, military and media.
Throughout history, we have seen numerous groups that have been dedicated to these reptilians: The Brotherhood of the snake, The dragon society, The Ophities .
There reptilians, possibly from the the Draco or Sirius star constellations, are believed to be able to shape-shift. This could be done at will or because it is difficult for them to hold their form in this dimension / planet / atmosphere. I personally believe that it is hard for them to hold their form and that the consumption of blood, specifically adrenochrome, somehow gives them power and aids them in maintaining their desired form. I believe that this has been the main reason for blood sacrifices throughout history; not only giving the blood directly to the reptilians, but also performing the blood sacrifices in homage to them.
AS MAINSTREAM MEDIA AS IT GETS OUTSIDE THE US, THIS ARTICLE FROM THE UK’S DAILY MAIL EXPLAINS, MATTER OF FACT, HOW PETER THIEL IS AN ADVOCATE OF CONSUMING YOUNG BLOOD, LITERALLY.
Peter Thiel believes blood transfusions from the young could be ‘biological fountain of youth’ and help people live forever
- Parabiosis involves the transfusion of blood plasma from a young donor
- Earlier studies have shown it to have anti-aging effects in animals
- And, this procedure has recently begun trials on human participants
CHEYENNE MACDONALD 1 AUGUST 2016
The blood of the young and healthy could one day serve as the ‘biological fountain of youth’ for those hoping to challenge the inevitability of death.
It may sound like vampirisim, but the bizarre practice known as ‘parabiosis’ has caught the attention of many life-extension enthusiasts – including billionaire tech investor Peter Thiel.
Early studies have shown that the procedure, which involves the transfusion of blood plasma from a young donor, can have age-reversing effects on the body, and it’s recently begun clinical trials on humans.

Studies have shown that parabiosis can have age-reversing effects on the body, and it’s caught the attention of many life-extension enthusiasts – including billionaire tech investor Peter Thiel, pictured
Human trials were launched recently by a company called Ambrosia, with participants paying $8,000 to get involved, according to Inc.
In the study, ‘Young Donor Plasma Transfusion and Age-Related Biomarkers,’ healthy individuals aged 35 or older receive a transfusion of blood plasma from donors younger than 25.
And, the firm has been contacted by Thiel Capital’s chief medical officer, Jason Camm, Inc. reports, who expressed interest in the work.
In an interview last year with Jeff Bercovici, Peter Thiel explained his interest in life-extension medicine.
Moving on from a discussion on caloric restriction, human growth hormone, and diabetes drug metformin, the investor said he isn’t yet convinced that scientists have found the cure-all technique.
But, he’s turned his sights to some ‘strangely underexplored’ fields.
‘I’m looking into parabiosis stuff, which I think is really interesting,’ Thiel told Bercovici.
‘This is where they did the young blood into older mice and they found that had a massive rejuvenating effect.’
Thiel went on to clarify that the procedure was of interest as a personal-health treatment, and said it would not require FDA approval.

The blood of the young and healthy could one day serve as the ‘biological fountain of youth. ’The bizarre practice known as ‘parabiosis’ involves the transfusion of blood plasma from a young donor, and has recently begun clinical trials on humans
Still, the investor remarked then that he hadn’t ‘quite, quite, quite started yet’ in moving forward with parabiosis, and according to Inc., this is still the case.
Scientists first experimented with parabiosis in the 1950s, and in recent years, it’s begun to gain attention once again.
In the organ systems of both animals and humans, the procedure has been found to reverse the symptoms of aging, though scientists don’t yet understand all of the mechanisms at work.
Jesse Karmazin, founder of Ambrosia, explains to Inc. that the blood of a young organism is rich with proteins that improve cell function, and can spur the production of these proteins in the recipient’s body.
And, the researcher says this effect appears to be ‘almost permanent.’
Below is a very long, detailed, researched article by the very Mainstream Media outlet, The Guardian. THIS IS A THING.
On an August morning in 2008, Tony Wyss-Coray sat in a conference room at the Veterans Affairs hospital in Palo Alto, California, waiting for his lab’s weekly meeting to begin. Wyss-Coray, a professor of neurology at Stanford University, was leading a young group of researchers who studied ageing and neurodegeneration. As a rule, the gatherings were forgettable affairs – the incremental nature of scientific progress does not lend itself to big surprises. But a lab member scheduled to speak that day had taken on a radical project, and he had new results to share.
Saul Villeda, an ebullient PhD student with slick black hair and a goatee, had spent the past year engrossed in research that called to mind the speculative medical science of the middle ages. He was investigating whether the old and frail could be rejuvenated by infusions of blood from the young. The hypothesis was not as absurd as it might sound.
Villeda’s work took skill. A mouse brain is the size of a peanut. To remove one for inspection is not difficult, but Villeda then had to cut each brain into wafers 1/25th of a millimetre thick using a cryomicrotome, a machine that resembles a benchtop deli slicer. Villeda took multiple slivers from about 40 mice and then stained them with a dye that binds to newborn neurons. Under a microscope the baby brain cells stand out like little brown trees.
The day before the lab meeting, Villeda and his colleague Kurt Lucin arrived early for work. With a small paintbrush, Villeda swept each brain slice, one after another, onto a microscope slide, and counted the tiny brown tree shapes. It took hours: he had about 200 slivers to inspect, from old and young mice. After totting up the newborn neurons in each section, he tapped the number into a statistics program. He finished after 10pm.
Though it was late, Villeda made Lucin stay with him to crunch the numbers. “It had been such a long experiment. I thought, if it doesn’t work, he’s here. We can go and grab a drink,” Villeda told me recently. He clicked a button on the screen marked “analyse”. The statistics program took all the data and calculated the average number of newborn neurons in the brains of each group of mice. A moment later, bar charts popped up on the screen.
Villeda got three hours’ sleep that night. The next morning, he stood up at the lab meeting and revealed to his colleagues what young blood did to the ageing brain. “There was a palpable electricity in the room,” Wyss-Coray recalled. “I remember seeing the images for the first time and saying, ‘Wow.’” Old mice that received young blood experienced a burst of brain cell growth in the hippocampus. They had three to four times as many newborn neurons as their counterparts. But that was not all: old blood had the opposite effect on the brains of young mice, stalling the birth of new neurons and leaving them looking old before their time.
The other scientists in the room were stunned. Some were sceptical. Could it be real? “This could be big,” said Wyss-Coray. “If an old mouse starts to make more neurons when you give it young blood? That is amazing.”
Since that meeting seven years ago, research on this topic has moved on dramatically. It has led some to speculate that in young blood might lie an antidote to the ravages of old age. But the apparent rejuvenating properties of young blood must be treated with healthy scepticism. The hopes they raise rest solely on mouse studies. No beneficial effects have ever been proven in humans. Then again, no one has ever looked.
That is about to change. In October 2014, Wyss-Coray launched the first human trial of young blood. At Stanford School of Medicine, infusions of blood plasma from young people are being given to older people with Alzheimer’s disease. The results are expected at the end of the year. It is the greatest test yet for the medical potential of young blood.
* * *
For much of history, people sought to halt ageing to achieve immortality – or at least to live for hundreds of years. These days, scientists tend to have more modest aims. In wealthy nations, basic healthcare and medical advances have driven up lifespan for the past century. Five years from now, for the first time in human history, there will be more over-60s than children under five years old. In 2050, two billion people will be 60 or older, nearly double the number today.
Behind that statistic lies a serious problem. People are living longer, but they are not necessarily living better. The old struggle with chronic conditions, often many at once: cancer, respiratory disease, heart disease, diabetes, arthritis, osteoporosis, dementia.

Medical researchers tend to tackle these diseases separately. After all, the illnesses are distinct: cancer arises from mutated DNA; heart disease from clogged up blood vessels; dementia from damaged brain cells. The biological processes that underpin the pathologies vary enormously. Each, then, needs its own treatment. Yet some researchers take another view: the greatest driver of disease in old age is old age itself. So why not invent treatments for ageing?
The idea has caught on, though it is still far from mainstream. Google’s secretive Calico operation, founded in 2013, is putting hundreds of millions of dollars into anti-ageing research. Craig Venter, the genetics entrepreneur, has launched a company called Human Longevity to find the genes that lead to long life. Meanwhile, scientists have asked the US Food and Drug Administration to approve trials of well-known drugs, such as the diabetes treatment, metformin, in the hope of uncovering anti-ageing effects.
Scientists may never halt the process entirely: ageing is an opaque and complex mingle of molecular pathways. But they might learn how to stop changes that underpin the worst chronic diseases. They want to extend healthspan, not lifespan. The stakes are enormous. Over the next decade, the cost of dementia care in Britain alone will rise to £24bn, a 60% increase on the cost in 2007. Last year, the World Health Organisation called the rise in chronic illness due to the greying population a major public health challenge.
Wyss-Coray is not the first person to wonder whether the answer to the problem of ageing might lie in human blood. One of the first physicians to propose blood transfusions to rejuvenate older people was Andreas Libavius, a German doctor and alchemist. In 1615 he proposed connecting the arteries of an old man to those of a young man. He had high hopes for the procedure. “The hot and spirituous blood of the young man will pour into the old one as if it were from a fountain of youth, and all of his weakness will be dispelled,” he claimed, in an account told in the Textbook of Bloodbanking and Transfusion Medicine by Sally Rudmann. It is unclear how it turned out; there is no record of the transfusion happening.

The fledgling years of the Royal Society, founded in London in 1660, witnessed some of the earliest experiments in blood transfusion. When Robert Boyle, one of the society’s founders, compiled a wishlist of scientific projects, the top entry was “The prolongation of life”. That might be achieved, he hoped, by replacing old blood with new.
Progress in science takes more than hope. With no knowledge of blood groups or coagulation factors, the early transfusion experiments were deadly. Before long, the procedure was banned, first in France, and then England. The pope endorsed the bans in 1679, and transfusion all but ceased for a century. When advances in medicine allowed its return, the emphasis was on healing the sick, not helping the aged.
It is 400 years since Libavius proposed that young blood could rejuvenate older people. At the time, the idea was radical and dangerous. Even though modern science has made blood transfusions safe, blood remains a mysterious fluid: it ferries more than 700 proteins and other substances around our bodies; many are known, but what they do is less clear. Wyss-Coray suspects that among them are factors that orchestrate the ageing process. If scientists can understand how they work, the ageing process might be laid bare. It could be slowed down, or perhaps even reversed.
* * *
When Wyss-Coray was in his 20s and 30s, he did not much care about ageing. “You have no understanding of what the problems are,” he recently told me, in his soft Swiss-German accent. Sitting in his office at the Veterans Affairs hospital, surrounded by books on immunology and biology, Wyss-Coray was fashionably unshaven, with a crop of blonde hair and lively blue eyes framed by dark rimmed glasses. “Now I see that the brain starts to slow down. I’m not as quick any more at grasping things, or remembering faces. I used to see a person for a few minutes and I’d remember their face. I couldn’t understand how they’d not remember who I was. And now it happens to me. It annoys the crap out of me.”
For Wyss-Coray, ageing has become much more than a personal bugbear. In 2014, the prestigious US journal, Science, named his work on young blood one of its breakthroughs of the year. He is regularly invited to give talks at conferences and the world’s top universities; in January, he spoke at the World Economic Forum in Davos. “In almost every talk I give, people make comments or jokes about vampires.” He slumped back in his chair and groaned. Another question also crops up: “I have people asking me, ‘Are you taking young blood?’” He assured me that he was not, and screwed up his face in horror, but it’s easy to see why they ask; he looks much younger than his 50 years.

Wyss-Coray was the first in his family to go to university. From the start, he set his sights on a career in the US. In 1993, he began as a postdoctoral fellow at the well-regarded Scripps Research Institute in La Jolla, California, studying HIV-related dementia. The work led to Alzheimer’s research, focusing on how the immune system played a role in the disease. In 2002, he joined Stanford University’s medical school, where he remains a faculty member.
Much of Wyss-Coray’s research on Alzheimer’s used mice that were genetically modified to develop the disease. This kind of experimentation has major limitations. Alzheimer’s mice mimic the forms of disease that run in families because of specific mutations, but they cannot tell us much about the origins of the sporadic forms of Alzheimer’s, which account for 99% of human cases. “People always joke: if you’re a mouse and you have Alzheimer’s, we can cure you, no problem,” Wyss-Coray told me. For humans, however, nothing so far has worked.
Frustrated by the limitations of his experiments, Wyss-Coray looked for better ways to understand how the disease first arose in humans. Brain scans and cognitive tests were out – neither revealed anything about disease at the molecular level. Nor would it make sense to study the brains of the dead, as scientists had traditionally done: the subtle neurological changes that lead to Alzheimer’s are set in motion two or three decades before patients are diagnosed, which meant old brains told you how bad the rot got, but not how the rot got started.
Wyss-Coray wondered if blood might hold the answer. Human blood travels 96,000 kilometres along the arteries, veins and capillaries of the circulatory system. It circulates through every organ. What if blood picked up information as it streamed around the body? What if its molecular makeup reflected the state of the brain, as it aged and changed with disease?
He assembled an international team of two dozen scientists to test the idea. They analysed blood plasma from more than 200 Alzheimer’s patients, and compared the profiles with those from healthy people. The findings, published in 2007, made headlines around the world. By measuring the levels of certain proteins in plasma, Wyss-Coray’s team believed they had found an accurate way to diagnose Alzheimer’s years before it began to take its toll. Wyss-Coray set up Satoris, a private company, to commercialise the research.
The study was too good to be true. Wyss-Coray’s later efforts to develop the test showed it was unreliable. In the course of this work, however, he had come across something intriguing. He noticed that in healthy people, the levels of certain proteins in blood fell with age. By 20 years old, most had already dropped steeply. Meanwhile, the levels of other proteins ramped up. Some doubled or tripled in old age. What the changes meant, no one knew.
* * *
One floor up from Wyss-Coray’s lab is the office of Thomas Rando, a neurologist and deputy director of the Stanford Center on Longevity. On his desk sits a small display of chemistry lab glassware and dozens of miniature figurines of the New York Giants. It was Rando who hired Wyss-Coray in 2002. “Tony is incredibly creative,” Rando told me. “He thinks about neuroscience in the context of the whole organism, as opposed to someone who has tunnel vision of the brain.”
In 2005, Rando oversaw a series of important experiments that would become closely intertwined with Wyss-Coray’s work. The question Rando wanted to investigate centred on stem cells. The body’s tissues need stem cells to remain healthy and in good working order, but in older people, stem cells stop doing their job – this is why wounds heal so much slower as we age. Rando wondered whether stem cells failed in old animals because they no longer got the right signals. What if something in young blood turned them back on again? Perhaps he could make older people heal as fast as young ones.
Rando’s experiments involved an unsettling but remarkable procedure in which mice were cut along the flanks and sewn together, wound-on-wound. This procedure, pioneered by the 19th-century French physiologist Paul Bert, is known as parabiosis. Bert’s work on conjoined rats demonstrated that, once their wounds had healed, the animals developed a single, shared circulatory system.
For a long time, experiments involving parabiosis were gruesome. In 1956, Clive McCay, an American gerontologist at Cornell University who was pursuing a similar line of research to Wyss-Coray, described his own attempts to conjoin rats in the Bulletin of the New York Academy of Medicine. “If the two rats are not adjusted to each other,” he wrote, “one will chew the head of the other until it is destroyed.” Grim though it was, McCay’s work hinted that young blood might have rejuvenating properties.
Though other scientists took up McCay’s experiments and got similarly encouraging results, the work was effectively abandoned in the 1970s. Not knowing what to make of their findings, researchers moved on to other projects. Only when parabiosis was resurrected at Stanford did scientists start to make sense of the anti-ageing effects.
Parabiosis is different today: ethics committees are strict and the surgical procedure has improved. The animals are genetically matched, so there is no risk of immune rejection. Once they have recovered from the operation, paired animals tend to eat normally and to make nests together. But the procedure is still disturbing – it would be a stretch to call the animals happy.
Scientists in Rando’s lab joined old and young mice for five weeks and looked at how well they repaired little tears in muscle tissue. The young blood activated stem cells in the old mice that swiftly regenerated their damaged muscles. The young mice, however, fared worse for their exposure to old blood. Their stem cells became sluggish, and their tissues healed more slowly. Rando saw hints of another effect too, but needed more evidence before he could publish: the old mice had begun to grow new brain cells.
The results led Wyss-Coray and Rando to collaborate. The kinds of proteins Wyss-Coray had seen rise and fall in blood were known to have effects on biological processes. What if they had driven the changes Rando had seen in muscle? Might they similarly revitalise the brain? Rather than being mere signatures of age, the proteins might be chemical cues for the ageing process itself.

Wyss-Coray asked his PhD student Saul Villeda to investigate. Villeda grew up in Pasadena on the outskirts of Los Angeles. His parents had immigrated illegally from Guatemala in the 1970s, and took jobs in factories, or as janitors. They became legal residents when Saul was a boy. Villeda had not planned on being a scientist when he went to college at the University of California in LA. But he enjoyed physiology classes: “I instantly fell in love with research,” he told me. “The idea that you were investigating something completely new and that you could come up with your own experiments to figure things out was amazing.” When he told his parents he wanted to be a university scientist, they didn’t really know what he meant. “I took them to my undergraduate lab to show them what a scientist looked like,” he said. “I think that really helped them understand.”
After Villeda presented his work on conjoined mice to Wyss-Coray at the lab meeting in August 2008, he went on to look at proteins in old and young blood. He found that the old mice, like old humans, had high levels of a protein called CCL11 in their blood. If you injected CCL11 into young mice, their learning and memory declined. The protein hampered the growth of new neurons. The young mice struggled to remember the location of a hidden platform in a water maze, and took longer to recognise a place where they had received a small but unpleasant electric shock. Villeda published the landmark research in 2011.
But the study failed to answer a major question: could proteins in young blood restore the mental capacities that old animals lost? Testing this was by no means easy. A mouse’s wits can be examined in a water maze, but two mice sewn together? It would be impossible to know how much one had led the other. Wyss-Coray believed that rather than experimenting with conjoined mice, the only option was to take blood from young mice, strip out the blood cells, and inject the plasma into old ones. This, too, was difficult. One mouse yields about 200 microlitres of plasma, the yellowish fluid that contains all the proteins. That is enough for two injections into another mouse. For an experiment that requires 10 injections into 10 old mice, you need to siphon the blood from 50 young mice.
Villeda was reluctant to do the experiment. He didn’t think it would work. But he changed his mind when he performed electrical measurements on slices of brain tissue and found that exposure to young blood strengthened the connections between neurons that had weakened in old mice. He went ahead with the plasma injections. Each mouse had one injection every three days for 24 days. The plasma came from three-month-old mice, the equivalent of human beings in their 20s, and went into 18-month-old mice, the equivalent of a human in their 60s.
The results were dramatic. Old mice given young plasma jabs aced the water-maze test, and quickly remembered the cage where they had earlier received an electric shock. They performed like mice half their age. “That time, I showed Tony the data one-on-one,” Villeda told me. “I was freaking out. I said: ‘I have to see this again.’”
Not everyone was impressed. The journal Nature rejected the study in 2012; its reviewers felt the work was not a big enough leap forward. So Wyss-Coray and Villeda sent it along to a sister publication, Nature Medicine. The editors there wanted to know precisely how young blood helped old mice. Villeda, who had just opened his own lab at the University of California in San Francisco, said he would find out.
Villeda looked at how young blood altered the way genes are expressed in old mice. He noticed a stark difference among genes that help neural connections strengthen and weaken, a process crucial for learning and memory. In normal ageing, the genes that control this “synaptic plasticity” become less active. Young plasma jabs ramped the gene activity back up again.
From the pattern of genes affected, Villeda traced the mechanism back to a master regulator in the brain, a protein known as CREB, which behaves like a switch that turns on many genes at once, and is instrumental in memory and learning from birth. To confirm young plasma was working through CREB, Villeda’s PhD student Kristopher Plambeck designed a virus that turned the master regulator off. When they injected the virus into old mice, young plasma had a much reduced effect on their brains. The animals performed better, but only slightly. It showed that young plasma worked through CREB, though not exclusively.
The study was published in Nature Medicine in 2014. Immediately, emails flooded in to Wyss-Coray’s inbox. Alzheimer’s patients wanted infusions of young blood. So did numerous aged billionaires. One, who flies around in a jet with his name emblazoned on the side, invited Wyss-Coray to an Oscars after-party this year. (He didn’t go.) Another correspondent wrote with a more disturbing offer: he said he could provide blood from children of whatever age the scientists required. Wyss-Coray was appalled. “That was creepy,” he said.
Wyss-Coray and Villeda were not the only scientists making headway in this area. Two members of the team behind Rando’s 2005 paper on stem cells had moved to the University of California, Berkeley, where they found that oxytocin, often called the love hormone, rejuvenated old muscle tissue. Another, Amy Wagers, had begun working at Harvard. She showed that when given young plasma, old mice regained their stamina. On a treadmill, the treated mice ran for an hour on average, compared with only 35 minutes for untreated ones.
Wagers picked out one factor, known as GDF11, as a rejuvenating protein in young blood. In Villeda’s most recent paper, published in July 2015, he found a second factor, B2M, which peaks in the blood of old mice, as it does in old humans: when injected into young mice, B2M impairs their memories.
The studies all point in one direction. Among the hundreds of substances found in blood are proteins that keep tissues youthful, and proteins that make them more aged. Wyss-Coray has a hypothesis: when we are born, our blood is awash with proteins that help our tissues grow and heal. In adulthood, the levels of these proteins plummet. The tissues that secrete them might produce less because they get old and wear out, or the levels might be suppressed by an active genetic programme. Either way, as these pro-youthful proteins vanish from the blood, tissues around the body start to deteriorate. The body responds by releasing pro-inflammatory proteins, which build up in the blood, causing chronic inflammation that damages cells and accelerates ageing.
“This opens an entirely new field. It tells us that the age of an organism, or an organ like the brain, is not written in stone. It is malleable. You can move it in one direction or the other,” says Wyss-Coray. “It’s almost mythological that something in young organisms can maintain youthfulness, and it’s probably true.”
* * *
As a business proposition, the transfusion of young blood raises all kinds of fears. It raises the spectre of a macabre black market, where teenagers bleed for the highest bidder, and young children go missing from the streets. Then there is the danger of unscrupulous dealers selling fake plasma, or plasma unsafe for human infusion. The fears are not unfounded: health has become one of the most lucrative sectors for criminals and con artists.
Havocscope, an online database, tracks the latest prices of all manner of black market goods and services. For $600 you can buy an AK-47 in Europe. A rhino-horn dagger will cost you $14,000. The services of a group of former military snipers? That will be $800,000. The list includes human organs too, mostly lungs, kidneys and livers. Today, a healthy seller can expect about $5,000 for their kidney. The organ broker who handles the deal can make a hefty profit, selling it on for $150,000 to a wealthy patient who needs a transplant.
In some countries, there is already a legal market for blood plasma. In the wake of the BSE crisis of the 1990s, plasma donations are not used in the UK. But in the US, donors can make $200 a month (plus loyalty points) from plasma donations. The fresh plasma is separated from the blood, and the red blood cells returned to the bloodstream, in a sitting that lasts 90 minutes. The plasma is used in medical procedures, to treat coagulation disorders and immune deficiencies. The business is completely legitimate, but if young plasma is proved to have anti-ageing effects, the risk of backstreet operators setting up will soar. When I asked Wyss-Coray if the prospect worried him, he looked serious. “Absolutely,” he said. “There are always going to be nutcases.”
These are worst-case scenarios. The Stanford trial may find that simply injecting young plasma into old people has little or no effect. Wyss-Coray confesses that he suspects as much. He believes that rejuvenating older people might take a more potent brew than natural plasma. He has in mind a concentrated blend of 10 or 20 pro-youthful factors from young blood, mixed with antibodies that neutralise the effects of ageing factors found in old blood.
In January 2014, Wyss-Coray set up Alkahest, a company that aims to separate plasma into its constituent parts, and combine them into a potent, rejuvenating cocktail. In Silicon Valley, scientists frequently launch start-up companies on the back of early-stage research – an alignment of the commercial and the scientific that some researchers still frown upon. Sergio Della Sala, a professor of human cognitive neuroscience at the University of Edinburgh, warns that creating a business before the science is done can raise a conflict of interest. “Science should first understand then sell,” he said. “We should always be skeptical when these two factors are reversed.”
Wyss-Coray formed Alkahest with Karoly Nikolich, an entrepreneur and neuroscientist at Stanford, who immigrated to the US from Hungary in the 1970s. I met Nikolich at his office in Menlo Park in February. He has thin hair, a full grey moustache and a mind filled with stories. Sat at a table on the sun-drenched roof terrace, Nikolich, handed me an Alkahest business card. The company logo is a blue droplet. Inside it is a golden disc.

Nikolich got to know Wyss-Coray in 2005. He had taken on the job of executive director of the Neuroscience Institute at Stanford University and over the years, Nikolich kept tabs on Wyss-Coray’s progress – from the Alzheimer’s blood test to the rejuvenating properties of young blood. But it wasn’t until spring 2012 that plans to form a company emerged. Nikolich had flown to Hong Kong to visit the family of Chen Din-hwa, a Chinese billionaire known as the King of Cotton Yarn. Three years earlier, Chen had died, aged 89, with Alzheimer’s disease. His grandson told Nikolich that towards the end of his life, Chen barely recognised his own family. Then he had a plasma transfusion for an unrelated condition, which seemed to have a spectacular effect. His mind was clearer. He was suddenly cogent.
Nikolich told them about Wyss-Coray’s research and the potential for plasma-based therapies that revitalised the ageing brain. Before long, the conversation turned to starting a company. The family invested a year later. The money got Alkahest established and ready to launch the first human trial of young plasma.
Alkahest’s ultimate goal – to identify the key proteins in plasma that rejuvenate or age human tissues and then manufacture a product that uses them – could take 10 to 15 years. In the near term, the company has another strategy. Earlier this year, the Spanish blood products firm, Grifols, pledged $37.5m for a 45% stake in Alkahest. With another $12.5m, the company will bankroll more research in exchange for rights to Alkahest’s first products. Over the next two years, Alkahest will take human plasma and divide it into fractions that are rich in different proteins. Each fraction will then be tested in mice to see if they boost brain function. Any that do will be swiftly introduced into human trials and developed into the first generation of products.
And what then? One enormous obstacle for hopes of plasma therapy is the limited supply. In a rough extrapolation from the mouse studies, Nikolich estimates that the globe’s entire plasma supply would be sufficient for only half a million of the world’s 15 million Alzheimer’s patients. “That means big questions about who gets treatment and who does not,” he said.
* * *
A short drive from the Palo Alto Veterans Affairs hospital is Stanford University’s School of Medicine, where the Alkahest trial is running. The woman in charge of the trial is Sharon Sha, a specialist in behavioural neurology who spends much of her time with patients who have Alzheimer’s. When I visited in February, Sha, a cheery woman with dark shoulder-length hair, was running late for a meeting in her third-floor office. But she was delayed for good reason: she had been infusing young plasma into an Alzheimer’s patient enrolled on the trial – a procedure that cannot be rushed.
The Alkahest trial is small. Sha can enrol only 18 people aged 50 to 90 with mild to moderate Alzheimer’s disease. Each receives a unit of young human plasma or saline once a week for four weeks. They have the next six weeks off, then have four more weeks of infusions. Those who had plasma first time around get saline and vice versa. The process is blinded, so neither the patients, nor their carers, nor Sha herself, know who is receiving what. Throughout the trial, doctors will look for cognitive improvements. Only at the end of the trial, as soon as October this year, will Sha analyse the findings.

Big questions lie ahead. Even if none of the patients benefit from young plasma, the research is far from finished. The plasma for the trial comes from donors under 30, and it may not be potent enough. The patients on the trial have dementia already, and may be too far gone to rescue.
Earlier this year, John Hardy of University College London, who is the most cited Alzheimer’s researcher in Britain, saw Wyss-Coray’s latest data at a meeting in London. “It’s really interesting work,” he told me. “It’s woken everybody up.” Nonetheless, Hardy is cautious; he suspects that young plasma will be less effective in people than in mice, because people live so much longer, and in far more varied environments. But, he said: “I would guess this will still point us towards pathways involved in ageing more generally.”
If patients improve with infusions of young plasma, scientists will be ecstatic. But the finding would need to be replicated, ideally at other hospitals, and in more patients, in order to convince researchers. If any benefits stand the test of time, the studies will move on, to tease out the best doses and ages at which to give plasma, how patients’ brains change, and whether improvements make a real difference to the life of someone who can no longer recognise their own family.
Then there is safety. Toying with the ageing process might backfire. Rando is concerned that pumping pro-youthful proteins into people for years could end up giving them cancer. Wyss-Coray agrees it is a worry, but points out that long-term growth hormone therapy appears to be safe. “We just don’t know yet whether or not it will be a problem,” he said.
Rando is more upbeat about infusing patients with pro-youthful proteins for short periods. An elderly person having surgery might get an infusion to help them heal like a teenager. “Let’s say it works. If you can target tissues and improve wound healing in older people, that would be a feasible approach. It would not be about making 90-year-olds younger, or having people live to 150. It’s about healthy living, not longer living,” he said.
In the 20 years that Wyss-Coray has lived in the US, his attitude to ageing has swung from disinterest to fascination. Why does a mouse live for three years and a human for 80? He sees its effects on a personal level too. He gets frustrated when a word fails to come as quickly as it once did, but knows how much worse it must be for people noticing the early signs of dementia: their words and memories slipping away into the gloom.
The carers of the patients enrolled in the young-blood trial keep journals to record how well the patients are doing. Among their pages may be signs of hope, that perhaps in the days after an infusion, a patient does a little bit better. “If it actually works? That would be huge. Every patient would want it,” Wyss-Coray said. He smiled. “I’d probably have to turn off my email and go somewhere else.”
Adrenochrome
FROM WIKIPEDIA, THE FREE ENCYCLOPEDIA
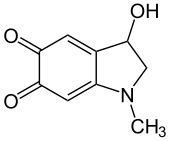
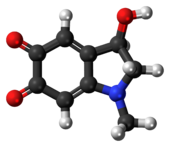
Adrenochrome is a chemical compound with the molecular formula C9H9NO3 produced by the oxidation of adrenaline (epinephrine). The derivative carbazochrome is a hemostatic medication. Despite a similarity in chemical names, it is unrelated to chrome or chromium.
CONTENTS
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-1-methyl-2,3-dihydro-1H-indole-5,6-dione
| |
| Other names
Adraxone; Pink adrenaline
| |
| Identifiers | |
3D model (JSmol)
| |
| ChemSpider | |
| ECHA InfoCard | 100.000.176 |
PubChem CID
| |
| Properties | |
| C9H9NO3 | |
| Molar mass | 179.18 g·mol−1 |
| Density | 3.264 g/cm³ |
| Boiling point | (decomposes, 115-120 °C) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Infobox references | |
CHEMISTRY[EDIT]
In vivo, adrenochrome is synthesized by the oxidation of epinephrine. In vitro, silver oxide (Ag2O) is used as an oxidizing agent.[1] Its presence is detected in solution by a pink color. The color turns brown upon polymerization.
EFFECT ON THE BRAIN[EDIT]
Several small-scale studies (involving 15 or fewer test subjects) conducted in the 1950s and 1960s reported that adrenochrome triggered psychotic reactions such as thought disorder, derealization, and euphoria.[2] Researchers Abram Hoffer and Humphry Osmond claimed that adrenochrome is a neurotoxic, psychotomimetic substance and may play a role in schizophrenia and other mental illnesses.[3] In what they called the “adrenochrome hypothesis”,[4] they speculated that megadoses of vitamin C and niacin could cure schizophrenia by reducing brain adrenochrome.[5][6] However, these hypotheses have never been scientifically accepted; adrenochrome is not currently believed to have any psychedelic properties.[7]
LAW[EDIT]
Adrenochrome is unscheduled by the Controlled Substances Act in the United States, but if sold as a supplement, sales must conform to U.S. supplement laws. If sold for consumption as a food or drug, sales are regulated by the FDA.[8][unreliable source?]
IN POPULAR CULTURE[EDIT]
- Author Hunter S. Thompson mentions adrenochrome in his book Fear and Loathing in Las Vegas. The adrenochrome scene also appears in the novel’s film adaptation. In the DVD commentary, director Terry Gilliam admits that his and Thompson’s portrayal is a fictional exaggeration. In fact, Gilliam insists that the drug is entirely fictional and seems unaware of the existence of a substance with even a similar name.
- The harvesting of an adrenal gland from a live victim to obtain adrenochrome for drug abuse is a plot feature in the second episode “Whom the Gods would Destroy”, of Series 1 of the British TV series Lewis (2008).[9]
- In Anthony Burgess‘ 1962 novel A Clockwork Orange, “drencrom” (presumably the Nadsat term for adrenochrome) is listed as one of the potential drugs that can be added to milk-plus (milk laced with a drug of the consumer’s choice).
- British gothic rock band The Sisters of Mercy released a song with the title Adrenochrome on the B side of their 1982 single Body Electric. Both songs were subsequently re-released on the compilation album Some Girls Wander by Mistake in 1992. The song refers to the substance multiple times.
SEE ALSO[EDIT]
- Appetite for Adrenochrome, the debut album by Sacramento, California pop-punk band the Groovie Ghoulies.
REFERENCES[EDIT]
- Jump up^ MacCarthy, Chim, Ind. Paris 55,435(1946)
- Jump up^ John Smythies (2002). “The adrenochrome hypothesis of schizophrenia revisited”. Neurotoxicity Research. 4 (2): 147–150. doi:10.1080/10298420290015827.
- Jump up^ Hoffer, A. Osmond, H., Smithies, J.; Schizophrenia: a new approach. Journal of Mental Science #100 (January, 1954)
- Jump up^ Hoffer, A (1990). “The Adrenochrome Hypothesis and Psychiatry”. Retrieved 2011-07-25.
- Jump up^ Hoffer, A. and Osmond, H. The Hallucinogens (Academic Press, 1967).
- Jump up^ Hoffer, A., Osmond, H., & Smythies, J. (1994). An Evolutionary Defense Against Severe Stress. Schizophrenia: A New Approach (pp. 205–221). Victoria, Canada: Journal of Orthomolecular Medicine
- Jump up^ “The controversy that these reports created just sort of died away, and the adrenochrome family has never been accepted as being psychedelic. No one in the scientific community today is looking in and about the area, and at present this is considered as an interesting historical footnote.” As seen at: Alexander Shulgin and Ann Shulgin (1991). “#157 (TMA)”. PiHKAL – A Chemical Love Story. Transform Press.
- Jump up^ Erowid. “Adrenochrome Law”. Retrieved 2013-01-14.
- Jump up^ “Inspector Lewis Series Synopsis”. Archived from the original on 2008-06-26.
EXTERNAL LINKS[EDIT]
- Adrenochrome Commentary at erowid.org
- Adrenochrome deposits resulting from the use of epinephrine-containing eye drops used to treat glaucoma from the Iowa Eye Atlas (searched for diagnosis = adrenochrome)
Parabiosis
[Interesting to note there is an episode of “Homeland” named “Parabiosis”.]
Parabiosis, meaning “living beside”, is a technical term in various contexts in fields of study related to ecology and physiology. It accordingly has been defined independently in at least three disciplines, namely experimental or medical physiology, the ecology of inactive physiological states, and the ecology of certain classes of social species that share nests.
CONTENTS
1ETYMOLOGY
ETYMOLOGY[EDIT]
Parabiosis derives most directly from new Latin,[1] but the Latin in turn derives from two classical Greek roots. The first is παρά (para) for “beside” or “next to”. In modern etymology, this root appears in various senses, such as “close to”, “outside of”, and “different”.
- In the physiological sense of “parabiosis”, “para” apparently was intended to mean “next to”.
- In describing transiently inactive physiological states, “para” apparently meant “outside of”.
- In ecological usage, the word was coined by the entomologist Auguste-Henri Forel as an analogue to “symbiosis“, also in the sense of “next to”. However, in this case, the emphasis was in contrast to “together” (“sym-“).
The second classical Greek root from which the Latin derives is βίος (bios), meaning “life”.
PARABIOTIC EXPERIMENTS[EDIT]
Left: A headless Cecropia Mothjoined with a pupa of the Polyphemus silkworm. Right: The abdomen of a Cecropia moth joined with a Cecropia pupa
In the field of experimental physiology, parabiosis is a class of techniques in which two living organisms are joined together surgically and develop single, shared physiological systems, such as a shared circulatory system.[2][3] Through surgically connecting two animals, researchers can prove that the feedback system in one animal is circulated and affects the second animal via blood and plasma exchange. Total blood volume is exchanged approximately ten times per day in rat experiments using parabiosis. One limitation of the experiments is that outbred rats cannot be used because it can lead to a significant loss of pairs due to intoxication of the blood supply from a dissimilar rat.[4]
In the mid-1800s, parabiotic experiments were pioneered by Paul Bert. He postulated that surgically connected animals could share a circulatory system. Bert was awarded the Prize of Experimental Physiology of the French Academy of Science in 1866 for his discoveries. Parabiotic experiments were scarcely revisited until the 20th century.[5]
Many of the parabiotic experiments since 1950 involve research regarding metabolism. One of these experiments was published in 1959 by G. R. Hervey in the Journal of Physiology. This experiment was to support the theory that damage to the hypothalamus, particularly the ventromedial hypothalamus, leads to obesity caused by the overconsumption of food. This results from the ventromedial hypothalamus failing to respond to physiological signals that suppress appetite. The result is attributed to the feedback control system in the brain. Rats in the study were from the same litter, which had been a closed colony for multiple years. The two rats in each pair had no more than 3% difference in weight. Rats were paired at four weeks old. Unpaired rats were used as controls. The rats were conjoined in three ways. First, the peritoneal cavities were opened and connected between the two rats. Later, to avoid the risk of tangling the two rats’ intestines together, smaller cuts were made. After more refinement of the experimental procedure, the abdominal cavities were not opened and the rats were conjoined at the hip bone with minimal cutting. In order to prove that the two animals were sharing blood, researchers injected dye into the veins of one rat and the pigment would show up in the conjoined rat. It was necessary to verify the exchange of blood and plasma. The scientists refined the lesion placement by practicing the procedure on other rats. The rats were killed with ether and weighed at the conclusion of the experiment and the amount of fat in each animal was quantified.
Many of the parabiotic pairs died throughout the experiment before its conclusion. In each pair, one rat became obese and exhibited hyperphagia. The weight of the rat with the surgical lesion rose rapidly for a few months, then reached a plateau as a direct result of the surgical procedure. After the procedure, the rat with the impaired hypothalamus voraciously ate and the paired rat decreased their appetite. The paired rat became obviously thin throughout the experiment, even rejecting food when it was offered. Some of the paired rats starved to death. Lesions subsequently made in the hypothalamus of two paired rats resulted in hyperphagia and obesity. That result verifies that the paired rat decreased its eating in a direct response to the signals in the blood from the rat with the lesion. If its brain was similarly altered, it also ate voraciously and became obese. The control rats who were given surgical lesions in the hypothalamus became similarly obese to the parabiotic counterparts.[6]
Later studies linked the effects of the previous parabiotic experiments about metabolism to the discovery of leptin. Many hormones and metabolites were proven to not be the satiety factor that caused one rat to starve in the experiments. Leptin seemed like a viable candidate. Starting in 1977, Ruth B.S. Harris, a graduate student under Hervey, repeated previous studies about parabiosis in rats and mice. Due to the discovery of leptin, she analyzed leptin concentrations of the mice in the parabiotic experiments. After injecting leptin into the obese mouse of each pair, she found that leptin circulated between the conjoined animals, but the circulation of leptin took some time to reach equilibrium. As a result of the injections, almost immediate weight loss resulted in the parabiotic pairs due to increased inhibition. Approximately 50–70% of fat was lost in the pairs. The obese mouse lost only fat. The lean mouse lost muscle mass and fat. Harris concluded that leptin levels are increased in the obese animal, but other factors could also affect the animals. Also, leptin was determined to decrease fat storage in both the obese and thin animals.[4]
Parabiotic experiments have also been used to study diabetes. Douglas Coleman did further parabiotic experiments to determine diabetic chromosomal relationships. A major gene that causes obesity in mice was identified on chromosome 6. The obese gene (ob/ob) was determined from one mutant mouse, discovered in 1950. Coleman and researchers further identified a gene on chromosome 4 that led to hyperphagia and obesity in mice. The gene also correlated to the severe onset of diabetes (db/db gene). The experimenters used parabiosis to conjoin a db/db mouse to a normal mouse, and the normal mouse would starve to death after one week. The db/db mouse would still have a high blood sugar and food in its system. The db/db mouse was determined to have a satiety factor so potent that the other mouse would starve to death. A db/db mouse was conjoined with an ob/ob mouse and the result was the same as the first experiment. The obese mouse starved in 20–30 days, similar to the thin counterpart in the first experiment. The db/db mutant mouse overproduced a satiety factor but could not respond to it, perhaps due to a defective receptor, whereas the ob/ob mutant recognized and responded to the factor but could not reproduce it. Further studies with db/db mice with lesions in the arcuate nucleus of the hypothalamus suggested that the receptor for the satiety factor was found in these brain receptors.[7]
Early parabiotic experiments also included cancer research. One study, published in 1966 by Friedell, studied the effects of radiation with X-rays on ovarian tumors. To study the tumors, two adult female rats were conjoined. The left rat was shielded and the right rat was exposed to high levels of radiation. The rats were given a controlled amount of food and water for the remainder of their natural lives. After death, the rats were autopsied and 149 of 328 pairs showed the presence of possible ovarian tumors in one or both of the two animals. This result matched previous studies of single rats. Since Friedell’s experiment, other parabiotic experiments have been useful in researching many types of cancer.[8]
Chronic diseases of age have been saluted as prime candidates for parabiotic research because of the potential to conjoin an older animal with a younger animal. This process could be used to research cardiovascular disease, diabetes, osteoarthritis, and Alzheimer’s disease. As animals age, their oligodendrocytes reduce in efficiency, resulting in decreased myelination, causing negative effects on the central nervous system (CNS). Julia Ruckh and fellow researchers have used parabiosis to study remyelination from adult stem cells to see if conjoining young with older mice could reverse or delay this process. In the experiment, the two mice were conjoined and demyelination was induced via injection into the older mice. The experiment determined that factors from the younger mice reversed CNS demyelination in older mice by revitalizing the oligodendrocytes. The monocytes from the younger mice also enhanced the ability of the older mice to clear myelin debris because the young monocytes can clear lipids from myelin sheaths more effectively than older monocytes. The conjoining of the two animals reversed the effects of age on the myelination cells. The ability of the young mouse’s cells was unaffected. Enhanced immunity from the younger mouse also promoted the general health of the older mouse in each pair. The results of this experiment could lead to therapy processes for people with demyelinating diseases like multiple sclerosis.[9]
Parabiotic research can be controversial. Due to accusations of animal cruelty, the practice is now shunned in many countries. Compared to other research techniques, there are relatively few parabiotic experiments. Some scientists argue for the benefits of parabiotic research. Eggel and Wyss-Coray argue that conjoining animals mimics naturally occurring parabiosis in nature due to the shared blood supply of conjoined twins. The experiments give insight into the way that bodily systems circulate, which has led to many advances in the study of a variety of diseases. Parabiotic experiments have been used to study obesity, chronic diseases of age, stem cell research, tissue regeneration, diabetes, transplants, tumor biology, and endocrinology.[5]
HUMAN TRIALS[EDIT]
A two-year human trial to evaluate the beneficial effects of infusions of plasma from young donors (16–25 years of age) using blood biomarkers was started in June 2016 in Monterey, California. A panel of age-associated biomarkers will be measured before and after treatment, representing a spectrum of physiologic pathways with evidence-based connections to aging.[10]
PARABIOSIS IN PHYSIOLOGY[EDIT]
Other than in experimental physiology, the term also is applicable to spontaneously occurring conditions such as in conjoined twins.[1]
Logically the word parabiosis would be equally applicable to various forms of parasitism such as the obligate parasitic reproduction of Anglerfish of the family Ceratiidae, in which the circulatory systems of the males and females unite completely. Without the attachment of males to a female, the endocrine functions cannot mature, the individuals fail to develop properly and die young and without reproducing.[11]
Similarly, in plants growing closely together roots or stems in intimate contact sometimes form natural grafts. More commonly, in parasitic plants such as mistletoe and dodder the haustoriaunite the circulatory systems of the host and the parasite so intimately that parasitic twiners such as Cassytha may act as vectors carrying disease organisms from one host plant to another.[12]
Inactive physiological states[edit]
Parabiosis as a term also applies to the states assumed by many organisms in various kingdoms of life, such as some bacteria, the bear animalcules (Tardigrada), and Rotifera. At least some members of all these taxa can survive drying out, often for decades or longer. Some bacteria, such as those causing anthrax, produce inactive, resistant spores that survive underground for many years. The eggs of some animals, such as Anostraca, the brine shrimps and their close relatives, have resistant shells and can survive in seasonally dried-out pools. In such a state the organisms show no sign of life until suitable conditions return. In this sense the para root apparently refers to the state as being “outside” of life.[1]
ECOLOGY OF SOCIAL ORGANISMS SHARING NESTS[EDIT]
Crematogaster modiglianii and Camponotus rufifemur ants sharing a nest
In the late 19th century the first examples were discovered, of colonies of ants that more or less routinely shared their nests with essentially unrelated species of ants. They did not obviously share anything beyond the upkeep of the nests, even segregating their brood, so these were a very surprising observations; most ants are radically intolerant of intruders, usually including even intruders of their own species.
In the early 20th century Auguste-Henri Forel coined the term “parabiosis” for such associations, and it was adopted by the likes of William Morton Wheeler.[13] The term has remained in currency, for example: “Parabiosis is defined as a special symbiosis, so far known only from a few Neotropical ant species, in which two or more species occupy the same nest site and forage together while keeping brood separate”.[14]
As is to be expected in matters concerning ethology and ecology however, such a class of behaviour patterns cannot be distinguished puristically from every other; there are exceptions and differences of kind and degree. Parabiosis ranges from the sharing of common trails to sharing common nests, and from effectively tolerating another species in general, to toleration only of the single colony of that species that shares the same nest. Furthermore, there is evidence for the partitioning of functions and unequal sharing of work between the two species in the nest.[15] Early reports that parabiotic ant colonies forage and feed together peacefully also have been qualified by observations that revealed ants of one species in such an association aggressively displacing members of the other species from artificially provided food, while also profiting by following their recruitment trails to new food sources.[14]
In practice parabiosis hardly could be a purely neutral interaction. There can be consequent benefits from shared nest defence and maintenance even when there is neither direct cooperation nor inimical interaction between the two associated populations in a nest.[16]
SEE ALSO[EDIT]
The Toll of Presidential Election 2016: Y’all Got Any More of That Adrenochrome? T.F.Y.M. When You’ve Drained All the Blood from the Third Rock from the Sun










































Comments
Post a Comment